Science Advance.
Science Advance. open online journal
© Illustration twirl
Physics
The spin and the Heisenberg principle
Melissa B. Blau
University of Tübingen, Tübingen 72076, Germany
Published: 2023-05-17
https://doi.org/10.59208/sa-2023-05-17-5
Eprint: ViYra: 2186.4771248
Abstract:
In 1921, on the basis of the experimental proof of the magnetic moment of the silver atom by Otto
Stern, it was postulated that electrons have a self-rotation impulse1. In the Gerlach-Stern experiment2 of 1922 the measurements of the angular momentum gave a much higher value than the theoretically calculated angular momentum value, which spoke for the then new theory of
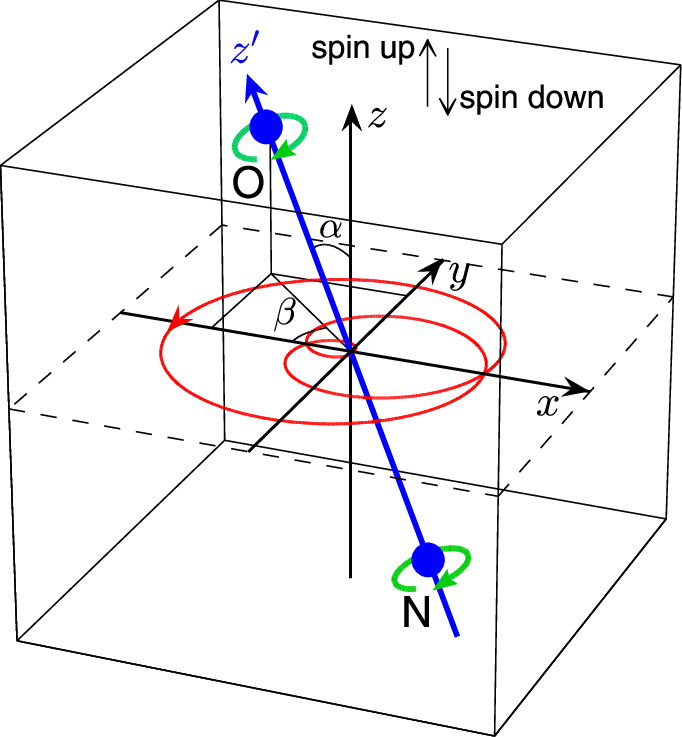
directional quantization, postulated in 1916 by Peter Debye and Arnold Sommerfeld. The physicists assumed a new undiscovered particle property, which they called spin. The fact that two successive measurements of one and the same particle were always independent of each other in terms of deflection could not be explained by the spin model and
initially remained a mystery. Since only a multiple of the half-integer spin was measured for matter particles such as electrons, protons and atomic nuclei as well as for photons and bosons, physicists assumed that the spin is quantized and can only assume certain quantum states. However, applying the Heisenberg priciple modified by Millette, the formula ∆L∆φ ≥ h/2 reveales that the measured constant spin of h/4π only gives only the term of the Heisenberg inequality, while the unquantized angular momentum might be much lower.
Cite this article
Blau M.B. The spin and the Heisenberg priciple. Science Advance (2023). https://doi.org/10.59208/sa-2023-05-17-5